What is the impact of the Ecosystem on fishery resources in the Bering Sea?
Patricia A. Livingston and Thomas K. Wilderbuer
NOAA Fisheries-Alaska Fisheries Science Center
Seattle, WA
Ecosystems consist of groups of interacting species joined together in a food web and the physical environment around them. Bering Sea fish production can be affected directly by the environment and indirectly through food web effects. The physical environment, or climate, can impact fish production through a variety of pathways. Direct effects of temperature on metabolism, growth and distribution of fish could occur. Food web effects could also occur through changes in the distribution and abundance of prey (zooplankton or forage fish in the pelagic food web; benthic invertebrates and fish in the benthic food web) or of predators and competitors (fish, marine mammals, and birds).
Temperature-induced changes in growth of a species might make a fish species grow more slowly if the temperature is outside that species' optimum temperature for growth. This could happen if the temperature is either too cold or too warm for a species. Reduced growth rates could also increase the vulnerability of some species to predation by keeping them at a smaller, more easily consumed size for longer periods.
Changes in the physical environment can influence the amount of food available to commercially exploited fish stocks. Weather changes might change the amounts of nutrients in the water, which might prevent phytoplankton blooms from occurring or change the type of phytoplankton species that blooms. Also, the timing of ice retreat can influence the timing of the phytoplankton bloom and whether zooplankton are present to consume it. If the bloom occurs when it is too cold for zooplankton, more phytoplankton falls to the bottom and can be used by benthic organisms such as polychaete worms and clams, which are consumed by crabs and flatfish that are part of the benthic food web. The timing of warmer-water phytoplankton blooms is closely matched with zooplankton blooms and may provide more food to the pelagic food web.
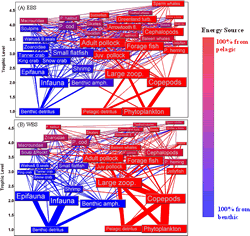 |
| The proportion of energy flow to ecosystem components that come from pelagic (red) or benthic (blue) sources on the eastern Bering Sea (EBS) and western Bering Sea (WBS) shelves (Aydin et al. 2002). |
The eastern (EBS) and western (WBS) Bering Sea food webs have both benthic and pelagic components (Aydin et al. 2002). One of the most dominant fish species in the eastern Bering Sea that is subjected to commercial fishing is the walleye pollock (Theragra chalcogramma). This species is a central part of the pelagic food web of the eastern Bering Sea and juveniles are heavily preyed upon by commercial and noncommercial fish, seabirds, and marine mammals. The benthic food web contains many commercially-utilized species of flatfish and crab such as yellowfin sole (Limanda aspera), northern rock sole (Lepidopsetta polyxystra), flathead sole (Hippoglossoides elassodon), red king crab (Paralithodes camtschaticus), snow crab (Chionoecetes opilio) and Tanner crab (Chionoecetes bairdi). Other flatfish such as the commercially exploited Greenland turbot (Reinhardtius hippoglossoides) and mostly nonexploited arrowtooth flounder (Atheresthes stomias) may live on the bottom but feed more on pelagic fish species such as walleye pollock. Other commercial species such as Pacific cod (Gadus macrocephalus) and Pacific halibut (Hippoglossus stenolepis) consume both benthic and pelagic prey.
Climate or weather changes are important in determining how many young of a fish species survive to adulthood. Many fish species reproduce by releasing many thousands of eggs into surface waters. If winds move these eggs and the young fish (larvae) that hatch from them into areas that are unfavorable for survival, either because there is no food for larvae, there are too many predators, or the water is too turbulent for fish larvae to successfully capture food, then that species may decline in abundance. If physical conditions favor the survival of a predator species, then the abundance of their prey may have increased mortality due to predation.
The extent and timing of the sea ice also determines the area where cold bottom water temperatures will persist throughout the following spring and summer. This eastern Bering Sea area of cold water, known as the cold pool, varies with the annual extent and duration of the ice pack and can influence fish distributions. Walleye pollock have shown a preference for warmer water and exhibit an avoidance of the cold pool (Wyllie-Echeverria 1995) such that in colder years they utilize a smaller portion of the shelf waters and in warm years have been observed as far north as the Bering Strait and the Chukchi Sea. Strong year-classes of pollock have been found to occur synchronously throughout the Bering Sea (Bulatov 1995) and coincide with above-normal air and bottom temperatures and reduced ice cover (Quinn and Niebauer 1995, Decker et al. 1995). These favorable years of production are the result of good juvenile survival and have been shown to be related to how much warm water habitat is present (Ohtani and Azumaya 1995) and the distribution of juvenile pollock relative to the adult population, which influences the level of predation (Wespestad et al. 2000). Warmer water also enhances rates of pollock egg development (Haynes and Ignell 1983), which may lead to increased survival.
Examination of the distributions of forage fishes including herring, capelin, eulachon and juvenile cod and pollock indicate temperature-related differences (Brodeur et al. 1999). Annual capelin distributions exhibit an expanded range in years with a larger cold pool and a contracted range in years of reduced ice cover. Although the productivity of capelin stocks in relation to temperature is not known, Bering Sea herring stocks exhibited improved recruitment during warm years (Williams and Quinn 2000) similar to herring stocks throughout their range where the timing of spawning has also been shown to be temperature related (Zebdi and Collie 1995).
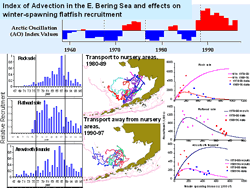
Recruitment responses of many Bering Sea fish and crab are linked to decadal scale patterns of climate variability (Francis et al. 1998; Hare and Mantua 2000; Zheng and Kruse 2000; Hollowed et al, 2001; Wilderbuer et al. 2002). Decadal changes in recruitment of some winter spawning flatfish species in the eastern Bering Sea (arrowtooth flounder, rock sole, and flathead sole) appears to be related to patterns seen in atmospheric forcing (Wilderbuer et al. 2002). The Arctic Oscillation, which tracks the variability in atmospheric pressure at polar and mid-latitudes, tends to vary between negative and positive phases on a decadal scale. The negative phase brings higher-than-normal pressure over the polar region and the positive phase does the opposite, steering ocean storms farther north. These patterns in atmospheric forcing in winter may influence surface wind patterns that advect fish larvae on or off the shelf . When the index was in its negative phase in the 1980s, southwesterly winds tended to dominate, likely transporting flatfish larvae to favorable nursery grounds. The positive phase in the 1990's showed winds to be more southeasterly, which would tend to advect larvae off-shelf.
However, periods of strong Aleutian Lows are associated with weak recruitment for some Bering Sea crab species and are unrelated with others (Zheng and Kruse 2000) depending on species-specific life history traits. Winds from the northeast favor retention of crab larvae in offshore mud habitats that serve as suitable nursery areas for young Tanner crabs that bury for protection (Rosenkranz et al. 2001). However, winds from the opposite direction promote inshore advection of crab larvae to coarse, shallow water habitats in inner Bristol Bay that serve as nursery areas for red king crabs that find refuge among biogenic structures (Tyler and Kruse 1998). Timing and composition of the plankton blooms may also be important, as red king crab larvae prefer to consume Thalassiosira spp. diatoms, whereas Tanner crab larvae prefer copepod nauplii.
Some species, such as Bering Sea herring, walleye pollock, and Pacific cod, show interannual variability in recruitment that appears more related to ENSO-driven climate variability (Williams and Quinn 2000; Hollowed et al. 2001). Years of strong onshore transport, typical of warm years in the Bering Sea, correspond with strong recruitment of walleye pollock, possibly due to separation of young fish from cannibalistic adults (Wespestad et al. 2000). Alaskan salmon also exhibit decadal scale patterns of production, which are inversely related to West coast salmon production patterns (Hare and Mantua 2000). Environmental variables such as sea surface temperature and air temperature significantly improved the estimates of productivity of Bristol Bay sockeye salmon compared to models containing only density-dependent effects (Adkinson et al. 1996).
These changes in abundance of predators and prey in the eastern Bering Sea affect food web relationships and thus, to some degree, the production of commercially important fish and crab that are prey for other members of the food web. As climate conditions provide increased abundance of predator species such as Pacific cod, halibut, and arrowtooth flounder, these predators may exert a greater degree of control over their prey populations. Multispecies modeling of predation on walleye pollock indicates that predation mortality on juvenile pollock varies across time, depending on the population levels of predators on juvenile pollock (Livingston and Jurado-Molina 2000), which include adult pollock. Hunt et al. (2002) hypothesize that predator control of pollock recruitment may occur periodically when the biomass of adult pollock is sufficiently large.
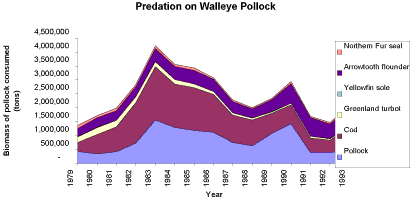 |
| Multispecies model estimates of the amounts of walleye pollock consumed by major predators in the eastern Bering Sea from 1979-1993 (Livingston and Jurado-Molina 2000). |
Scientists are working to improve understanding of the relative importance of the direct effects of the environment and the indirect food web effects on Bering Sea fish production. Research is being conducted that is searching for the links between physical environmental changes and biological production. Improving our observation system of the physical environment and lower trophic level production (i.e., phytoplankton and zooplankton) will be important in this endeavour. Prediction of ecosystem effects on fishery resource production will also require more detailed models that link physical environment and marine resource production.
References:
Adkinson, M.D., R.M. Peterman, M.F. Lapointe, D.M. Gillis, and J. Korman. 1996. Alternative models of climatic effects on sockeye salmon, Oncorhynchus nerka, productivity in Bristol Bay, Alaska, and the Fraser River, British Columbia. Fish. Oceanogr. 5: 137-142.
Aydin, K.Y., V.V. Lapko, V.I. Radchenko, and P.A. Livingston. 2002. A comparison of the eastern Bering and western Bering Sea shelf and slope ecosystems through the use of mass-balance food web models. U.S. Dep. Commerce, NOAA Tech. Memo. NMFS-AFSC-130. 78p.
Bulatov, O. A. 1995. Biomass variation of walleye pollock of the Bering Sea in relation to oceanological conditions. In: Beamish, R. J. (Ed), climate change and northern fish populations, Canadian Special Publication in Fisheries and Aquatic Science, Vol. 121, pp. 631-640.
Brodeur, R. D., Wilson, M. T., Walters, G. E., and Melnikov, I. V. 1999. Forage fishes in the Bering Sea: distribution, species associations, and biomass trends. In Dynamics of the Bering Sea, pp. 509-536. Ed. By T. Loughlin, and K. Ohtani. University of Alaska Sea Grant, fairbanks. 825 pp.
Decker, M. B., Hunt Jr, G. L., Byrd Jr., G.V. 1995. The relationships among sea surface temperature, the abundance of juvenile walleye pollock, and the reproductive performance and diets of seabirds at the Pribilof Islands, southeastern Bering Sea. In: Beamish, R. J. (Ed), climate change and northern fish populations, Canadian Special Publication in Fisheries and Aquatic Science, Vol. 121, pp. 425-437.
Francis, R.C., S.R. Hare, A.B. Hollowed, W. S. Wooster. 1998. Effects of interdecadal climate variability on the oceanic ecosystems of the NE Pacific. Fish. Oceanogr. 7:1-21.
Hare, S.R. and N.J. Mantua. 2000. Empirical evidence for North Pacific regime shifts in 1977 and 1989. Progr. Oceanogr. 47:103-145.
Haynes, E.G. and S.E. Ignell. 1983. Effect of temperature on rate of embryonic development of walleye pollock (Theragra chalcogramma). Fisheries Bulletin 81, 890-894.
Hollowed, A.B., S.R. Hare, and W.S. Wooster. 2001. Pacific Basin climate variability and patterns of Northeast Pacific marine fish production. Progr. Oceanogr. 49:257-282.
Hunt, G.L., P. Stabeno, G. Walters, E. Sinclair, R.D. Brodeur, J.M. Napp, N.A. Bond. 2002. Climate change and control of the southeastern Bering Sea pelagic ecosystem. Deep See Research Part II. 49: 5821-5853.
Livingston, P.A. and J. Jurado-Molina. 2000. A multispecies virtual population analysis of the eastern Bering Sea. ICES J. Mar. Sci. 57:294-299.
Ohtani, K., and T. Azumaya. 1995. Influence of interannual changes in ocean conditions on the abundance of walleye pollock in the eastern Bering Sea, pp. 87-95. In R. J. Beamish [ed.] Climate change and northern fish populations. Can. Spec. Publ. Fish Aquat. Sci. 121.
Quinn, T.J. II, and H. J. Niebauer. 1995. Relation of eastern Bering Sea walleye pollock (Theragra chalcogramma) recruitment to environmental and oceanographic variables, p. 497-507. In R. J. Beamish [ed.] Climate change and northern fish populations. Can. Spec. Publ. Fish. Aquat. Sci. 121.
Rosenkranz, G.E., A.V. Tyler, and G.H. Kruse. 2001. Effects of water temperature and wind on recruitment of Tanner crabs in Bristol Bay, Alaska. Fisheries Oceanography 10: 1-12.
Tyler, A.V., and G.H. Kruse. 1998. A comparison of year-class variability of red king crabs and Tanner crabs in the eastern Bering Sea. Memoirs of the Faculty of Fisheries, Hokkaido University, Vol. 45, No. 1: 90-95, Hakodate, Japan.
Wespestad, V.G., L.W. Fritz, W.J. Ingraham, and B.A. Megrey. 2000. On relationships between cannibalism, climate variability, physical transport, and recruitment success of Bering Sea walleye pollock (Theragra chalcogramma). ICES J. Mar. Sci. 57: 272-278.
Wilderbuer, T.K., A.B. Hollowed, W.J. Ingraham, P.D. Spencer, M.E. Conners, N.A. Bond, and G.E. Walters. 2002. Flatfish recruitment response to decadal climate variability and ocean conditions in the eastern Bering Sea. Progr. Oceanogr. 55:235-247.
Williams, E.H. and T.J. Quinn, II. 2000. Pacific herring, Clupea pallasi, recruitment in the Bering Sea and north-east Pacific Ocean, II: relationships to environmental variables and implications for forecasting. Fish. Oceanogr. 9:300-315.
Wylllie-Echeverria, T., 1995. Sea-ice conditions and the distribution of walleye pollock (Chalcogramma theragra) on the Bering and Chukchi shelf. In: Beamish, r. J. (Ed.), Climate change and northern fish populations, Canadian Special Publication in Fisheries and Aquatic Science, Vol. 121, pp. 131-136. National research Council of Canada Ottawa.
Zebdi, A. and J. S. Collie. 1995. Effect of climate on herring (Clupea pallasi) population dynamics in the Northeast Pacific Ocean. In R. J. Beamish [ed.] Climate change and northern fish populations. Can. Spec. Publ. Fish. Aquat. Sci. 121.
Zheng, J. and G.H. Kruse. 2000. Recruitment patterns of Alaskan crabs in relation to decadal shifts in climate and physical oceanography. ICES J. Mar. Sci. 57:438-451.